New Study Reveals IgM Antibodies Act as Mechanical Stabilizers Against Bacteria
This paradigm-shifting finding redefines our understanding of how antibodies defend the body and could pave the way for next-generation antibody-based therapies.
- Country:
- India
In a groundbreaking discovery, scientists from the S.N. Bose National Centre for Basic Sciences (SNBNCBS), an autonomous institute under the Department of Science and Technology (DST), have revealed a surprising new property of antibodies. The study shows that Immunoglobulin M (IgM) – the largest antibody in our immune system – does not just act like a chemical key fitting into microbial locks, but also functions like a mechanical engineer, stabilizing bacterial proteins to neutralize them.
This paradigm-shifting finding redefines our understanding of how antibodies defend the body and could pave the way for next-generation antibody-based therapies.
Antibodies: Beyond Chemical Locks and Keys
Traditionally, antibodies are understood as molecules that recognize and bind to pathogens, neutralizing them through highly specific "lock-and-key" chemical interactions. Each antibody type – IgG, IgA, IgE, IgD, and IgM – has specialized roles in defending the body.
Among them, IgM is the largest and usually the first responder during infections. Unlike smaller antibodies, IgM has multiple binding sites, allowing it to form complex structures with microbes. The new study demonstrates that this unique structure gives IgM a previously unrecognized mechanical ability to stabilize dangerous proteins, preventing them from unfolding and harming host cells.
The Study: Focus on Protein L
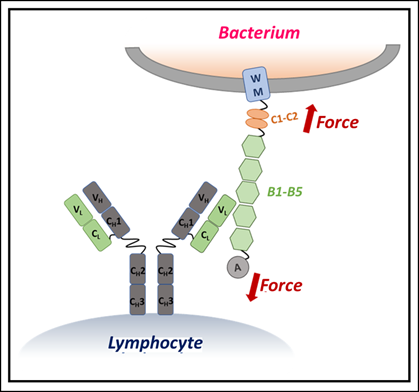
The researchers investigated Protein L, a superantigen produced by the bacterium Finegoldia magna. Protein L binds to the light chains of antibodies on B lymphocytes in unusual ways, sometimes aiding bacteria in evading immune responses.
What makes the study remarkable is the use of single-molecule force spectroscopy – a cutting-edge technique that applies ultra-precise mechanical forces to individual molecules, mimicking the shear stresses proteins encounter inside the human body (such as blood flow or immune cell attack).
The scientists observed that when IgM binds to Protein L, it dramatically increases the mechanical stability of the bacterial protein. In essence, IgM works like a molecular brace, stiffening Protein L and making it resistant to unfolding under mechanical stress.
Concentration and Binding Effects
The team discovered a concentration-dependent effect:
-
Higher amounts of IgM led to greater stabilization of Protein L.
-
Computer simulations revealed that IgM’s multiple binding sites allowed it to attach to Protein L at several points simultaneously, creating a synergistic stabilizing effect.
-
By contrast, smaller antibodies lacked this mechanical reinforcement capability.
This means that IgM does not just bind pathogens chemically—it can also modify their physical behavior, altering how they withstand forces in the human body.
Implications for Bacterial Infections
Many bacteria experience mechanical forces inside the body, such as those caused by fluid flow in the bloodstream, mucosal environments, or immune cell activity. By mechanically stiffening bacterial toxins and virulence factors, IgM may effectively neutralize them without necessarily destroying them chemically.
This introduces a completely new dimension to how antibodies protect us: as mechanical modulators that prevent harmful proteins from functioning.
A New Frontier in Antibody-Based Therapies
The discovery opens promising avenues for biomedical innovation:
-
Therapeutic Antibodies: Future drugs could be designed to mimic IgM’s stabilizing power, creating therapies that disarm pathogens by mechanically stiffening their toxins.
-
Next-Gen Vaccines: Insights into IgM’s behavior could inform vaccine design, enhancing immune responses by leveraging mechanical stabilization.
-
Synthetic Biology Applications: Engineered antibodies with mechanical properties could be deployed in combating antimicrobial resistance, offering an alternative to traditional antibiotics.
This aligns with global efforts to develop novel antimicrobial strategies in the face of rising drug-resistant infections.
Expert Reflections
Dr. Chandra Sekhar Pemmasani, Minister of State for Communications and Rural Development, recently highlighted India’s growing strength in applied biosciences and biotechnology. Discoveries like this reinforce the country’s scientific leadership and showcase how multidisciplinary research—combining physics, biology, and immunology—can yield transformative insights.
The SNBNCBS team emphasized that their study highlights an underappreciated dimension of immunity: antibodies as mechanical stabilizers. This challenges the conventional view of antibodies as merely chemical binders and broadens our understanding of their role in the complex fight against disease.
This study positions IgM as more than a chemical defender – it is a molecular engineer capable of reshaping pathogen proteins under mechanical stress. As India’s scientists continue to push the boundaries of biomedical research, such discoveries could lay the foundation for innovative therapies against bacterial infections, ultimately safeguarding human health in new and unexpected ways.










