AI promises faster, more reliable detection of diabetic foot infections
AI models can act as a valuable "second reader" in clinical settings, improving early detection rates and supporting clinicians in decision-making. By embedding advanced algorithms into diagnostic workflows, healthcare providers can potentially save lives while reducing the burden on specialists.
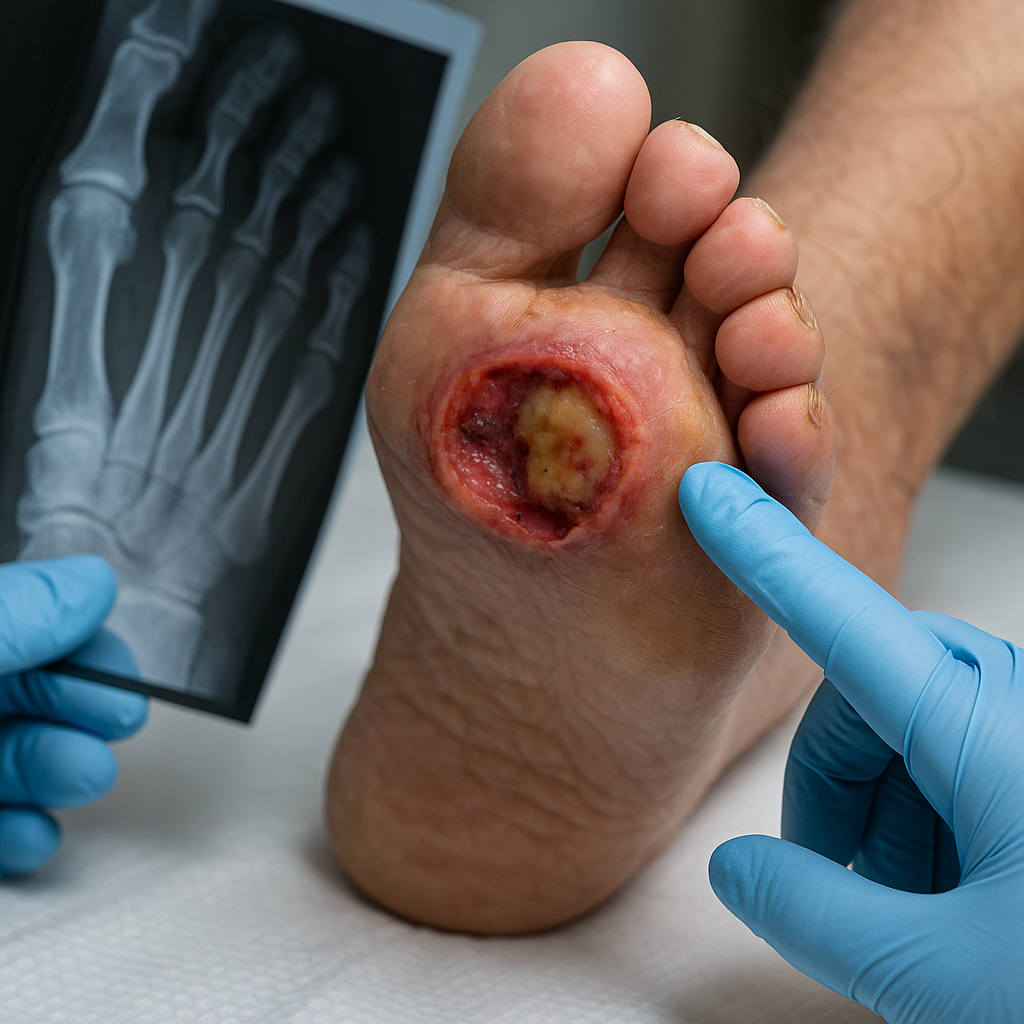
Diabetic foot osteomyelitis (DFO), a severe complication of diabetic foot ulcers (DFUs), often leads to prolonged infections, amputations, and increased healthcare costs. Early and accurate diagnosis is critical to prevent these outcomes, yet conventional radiograph interpretation depends heavily on clinician expertise and is prone to variability.
Researchers have now developed a deep learning (DL) model capable of detecting DFO from standard radiographs. Their findings, published in a paper titled "Using Artificial Intelligence for Detecting Diabetic Foot Osteomyelitis: Validation of Deep Learning Model for Plain Radiograph Interpretation" in Applied Sciences, validates a deep learning model designed to detect DFO from standard radiographs. This innovation could transform the diagnostic process, particularly in settings where expert evaluation is limited.
Why early detection of DFO is critical
DFO is a life-threatening condition affecting diabetic patients with foot ulcers, requiring prompt detection to avoid serious outcomes. Standard diagnostic pathways rely on a combination of clinical examination, radiographic interpretation, and sometimes invasive bone biopsy for confirmation. However, radiographs alone are often insufficient due to overlapping signs with other conditions, leading to missed or delayed diagnoses.
Early intervention is crucial to reduce complications, minimize hospitalization, and prevent amputations. Traditional methods, while effective in skilled hands, are not uniformly accessible. In many healthcare systems, radiographs are read by non-specialists or in high-volume environments where errors may occur. Here, artificial intelligence offers a potential solution by providing consistent, rapid, and sensitive diagnostic support.
The authors highlight that AI models can act as a valuable "second reader" in clinical settings, improving early detection rates and supporting clinicians in decision-making. By embedding advanced algorithms into diagnostic workflows, healthcare providers can potentially save lives while reducing the burden on specialists.
How the AI model performs compared to clinicians
The research team developed a deep learning model based on the ResNet-50 convolutional neural network, training it with radiographs from 168 patients with DFUs and suspected osteomyelitis. Histopathological analysis of bone biopsies served as the diagnostic gold standard, ensuring rigorous validation.
Results show the AI model achieved a sensitivity of 92.8%, outperforming the clinician’s sensitivity of 90.2%. The model also demonstrated a positive predictive value (PPV) of 0.97, indicating strong reliability in detecting positive cases. This high sensitivity is particularly important in preventing missed diagnoses, which can have devastating consequences in diabetic patients.
However, the model exhibited low specificity at 4.4%, compared to the clinician’s 37.8%. This means the AI tended to generate more false positives, flagging cases as DFO even when they were not. While this could lead to unnecessary follow-up tests, in high-risk environments such as diabetic foot clinics, the authors argue that it is preferable to over-diagnose than to miss critical cases.
Agreement between the AI and the clinician was weak, with a Cohen’s kappa coefficient of −0.105. Despite this, the model successfully identified 81.5% of positive cases that clinicians also diagnosed, confirming its utility as an early detection tool. The authors conclude that while AI cannot replace expert judgment, it can enhance diagnostic confidence, particularly in ambiguous cases.
The model was also integrated into a web-based interface, allowing clinicians to upload radiographs, annotate lesions, and receive immediate diagnostic feedback. This user-friendly platform demonstrates the feasibility of incorporating AI into routine clinical workflows without significant disruption.
What challenges remain before AI integration in clinical practice?
Despite promising results, the study notes several limitations that must be addressed before widespread adoption. The model’s low specificity poses a challenge, as over-diagnosis could strain healthcare resources with unnecessary tests and treatments. Improving the algorithm to better differentiate true positives from false positives will be critical.
The retrospective nature of the research is another limitation, as the model was validated on historical data rather than real-time clinical cases. Prospective studies are necessary to confirm its performance under routine conditions. Additionally, the AI did not incorporate other clinical information, such as probe-to-bone tests or laboratory markers, which could enhance accuracy when combined with radiographic data.
The authors suggest that future improvements should focus on integrating multimodal data, refining the model to reduce false positives, and conducting trials across diverse clinical environments. They also stress the importance of regulatory approval and clinician training to ensure AI tools are used appropriately and effectively.
AI tools should not replace clinicians but instead serve as decision-support systems, particularly in settings with limited access to specialists. By offering an additional layer of analysis, AI can help clinicians prioritize high-risk cases, improve diagnostic accuracy, and optimize patient outcomes, the study asserts.
- FIRST PUBLISHED IN:
- Devdiscourse










