AI cuts hours from viral testing, saving biopharma firms weeks of work annually
The production of vaccines, monoclonal antibodies, and recombinant proteins relies on mammalian cell cultures, which are vulnerable to contamination by retroviruses such as murine leukemia virus and xenotropic MLV. Detecting these contaminants is not optional; it is a regulatory requirement for ensuring patient safety.
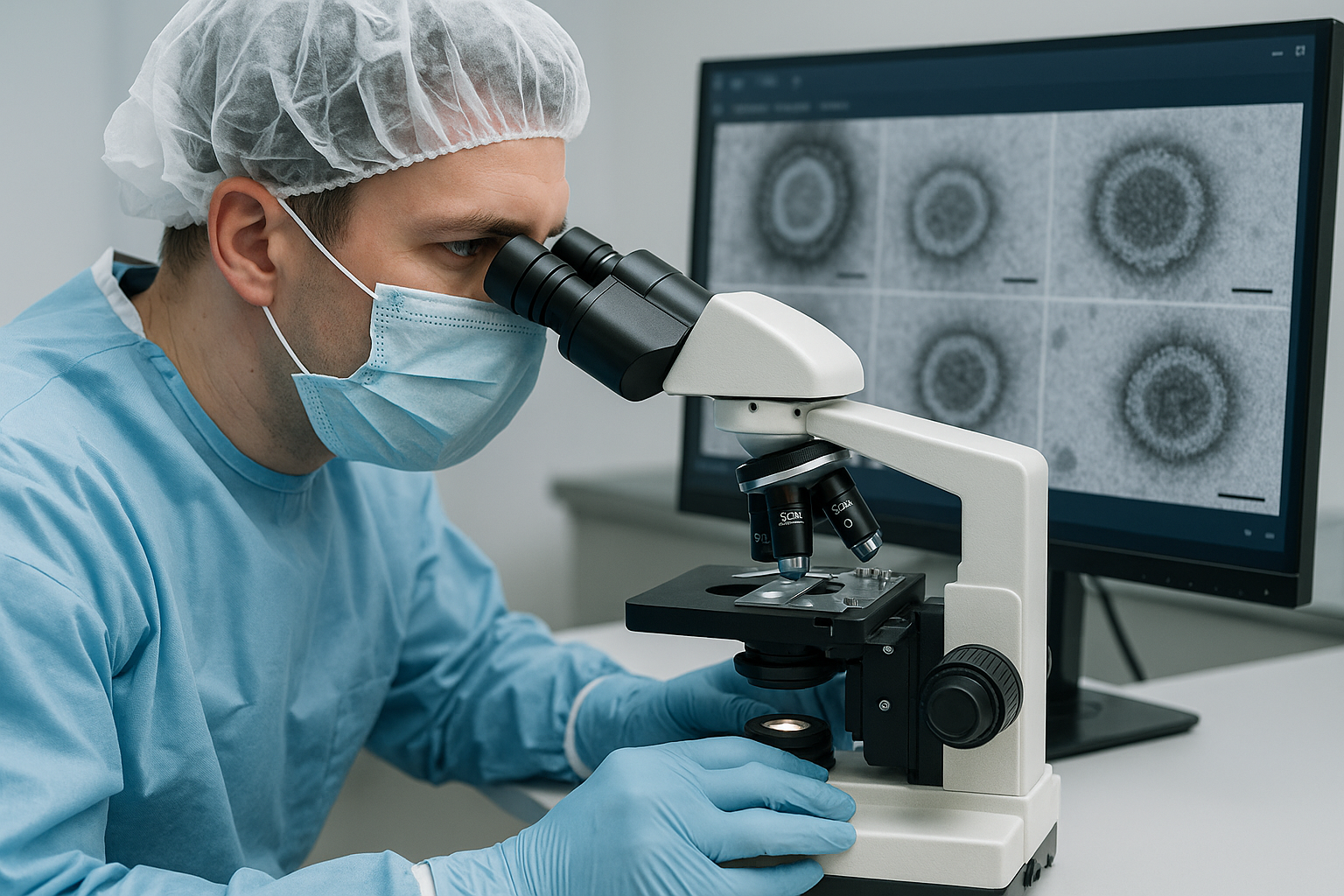
A team of researchers has developed an artificial intelligence–powered system that could dramatically shorten the time required for viral safety testing in the biopharmaceutical industry. Their work, published in Applied Sciences, addresses one of the most pressing challenges in drug manufacturing: the slow and error-prone process of identifying viral contaminants in mammalian cell cultures used to produce vaccines and therapeutic proteins.
The study, titled “AI-Enhanced Virus Detection in Biopharmaceutical Production Processes,” details how the researchers enhanced the YOLOv11 deep learning model to deliver near-perfect accuracy in detecting viruses in unprocessed bulk samples, setting the stage for faster, more reliable quality control in a heavily regulated industry.
Why viral safety testing is critical in biopharmaceuticals
The production of vaccines, monoclonal antibodies, and recombinant proteins relies on mammalian cell cultures, which are vulnerable to contamination by retroviruses such as murine leukemia virus and xenotropic MLV. Detecting these contaminants is not optional; it is a regulatory requirement for ensuring patient safety.
Until now, the gold standard has been transmission electron microscopy (TEM), which allows direct visualization of viral particles. While TEM provides critical confirmation, it is also notoriously time-consuming. Each batch can take two to four hours to analyze, requiring skilled specialists to sift through images manually. Fatigue, subjectivity, and the sheer workload all increase the risk of missed detections, delays, and compromised quality assurance.
The authors note that with biopharmaceutical companies often producing hundreds of batches each year, these delays add up, increasing costs and slowing down time-to-market for essential therapies. The challenge has been finding a solution that not only accelerates testing but also maintains the level of accuracy demanded by regulators.
How artificial intelligence enhances virus detection
To address these bottlenecks, the research team introduced an improved YOLOv11-based model tailored specifically for single-class virus detection in TEM images. The model incorporates specialized modules, C3K2, SPPF, and C2PSA in the backbone, with BiFPN and C3K2_IDWC added to handle irregular viral morphologies. These enhancements allow the algorithm to identify viral particles with greater precision while maintaining efficiency.
The system achieved striking results: a mean average precision of 0.995, recall of 1, and precision of 0.954. Compared to the baseline YOLOv11n, the model reduced parameters by over 33 percent while actually improving accuracy. The researchers report that their optimized model outperformed other state-of-the-art algorithms such as YOLOv8n and YOLOv10n across both accuracy and computational efficiency benchmarks.
Each image was processed in less than one second, cutting the analysis time of a typical TEM dataset from hours to minutes. For manufacturers processing 100 batches annually, this translates into a savings of nearly 50 working days of labor each year. Such gains have clear financial implications but also improve consistency by removing human variability from the detection process.
The team also tested the model under simulated real-world conditions. Even when Gaussian blur noise was introduced to replicate common preparation artifacts, the system maintained a perfect recall rate, ensuring no viral particles went undetected. Although precision dropped slightly due to false positives, the robustness of the model demonstrated its potential for deployment in actual manufacturing environments.
What challenges remain before full adoption?
Despite its promise, the study acknowledges that limitations must be addressed before the system can be widely adopted. The dataset used in training consisted of 195 TEM images, which, although carefully annotated, represents a relatively small pool for machine learning. Expanding the dataset to include a wider variety of viruses, morphologies, and cell lines will be crucial to validate the model across the diverse contexts of biopharmaceutical production.
The authors also stress the importance of extending validation beyond Chinese hamster ovary (CHO) cell lines, which currently dominate biopharmaceutical manufacturing but do not represent all use cases. Further testing across different host systems will be necessary to meet regulatory requirements and gain industry trust.
Noise and artifact variability in TEM imaging pose another challenge. The authors suggest integrating preprocessing modules that reduce background interference and potentially adding an impurity classification branch to help distinguish viral particles from debris. These improvements could help minimize false positives while retaining the model’s strong recall performance.
While the model holds potential for regulatory acceptance, adoption will depend on demonstrating interpretability, reproducibility, and compliance with strict industry standards. This means collaboration with regulators, standardized validation protocols, and possibly third-party auditing to ensure that AI-driven decisions meet the same scrutiny as traditional methods.
- FIRST PUBLISHED IN:
- Devdiscourse










